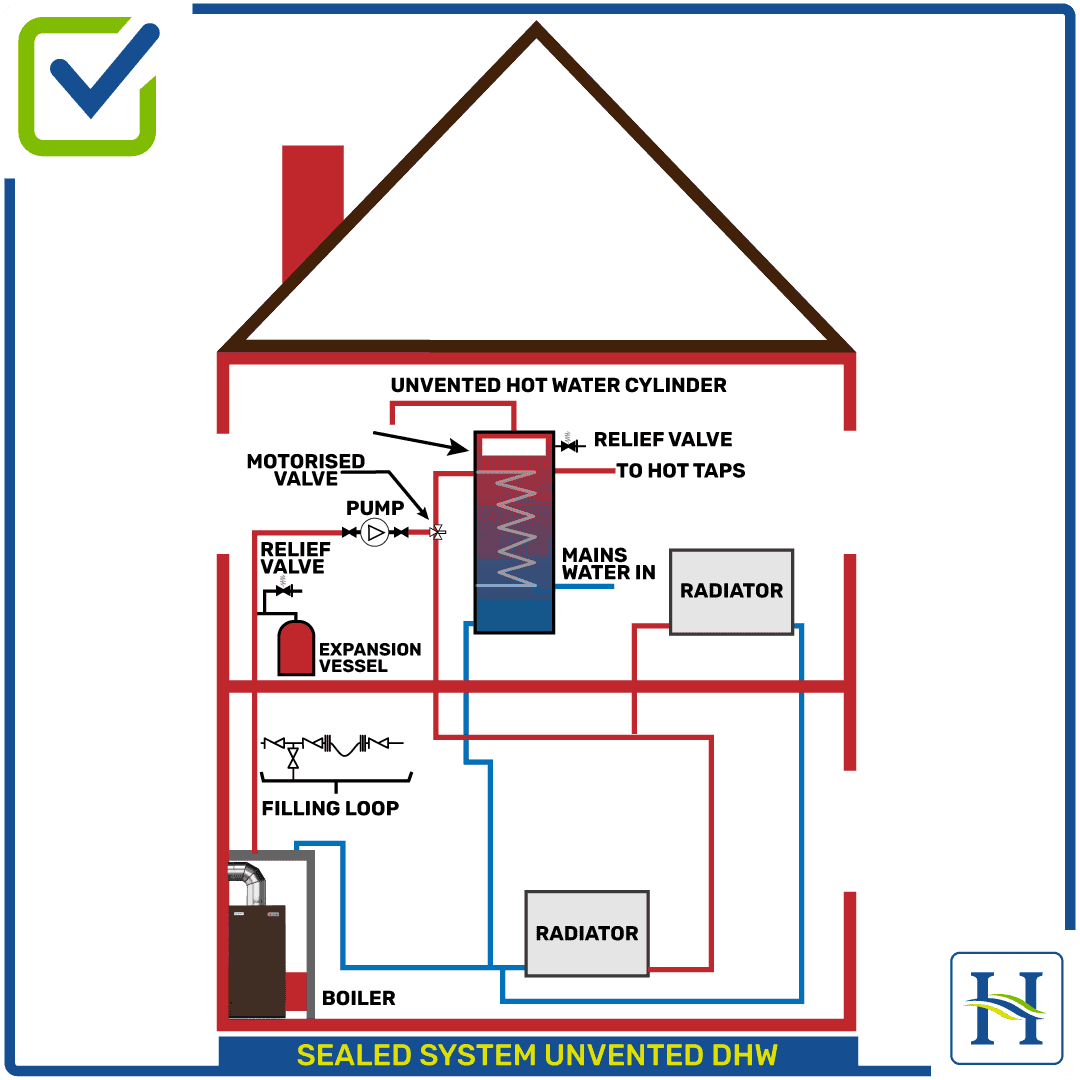
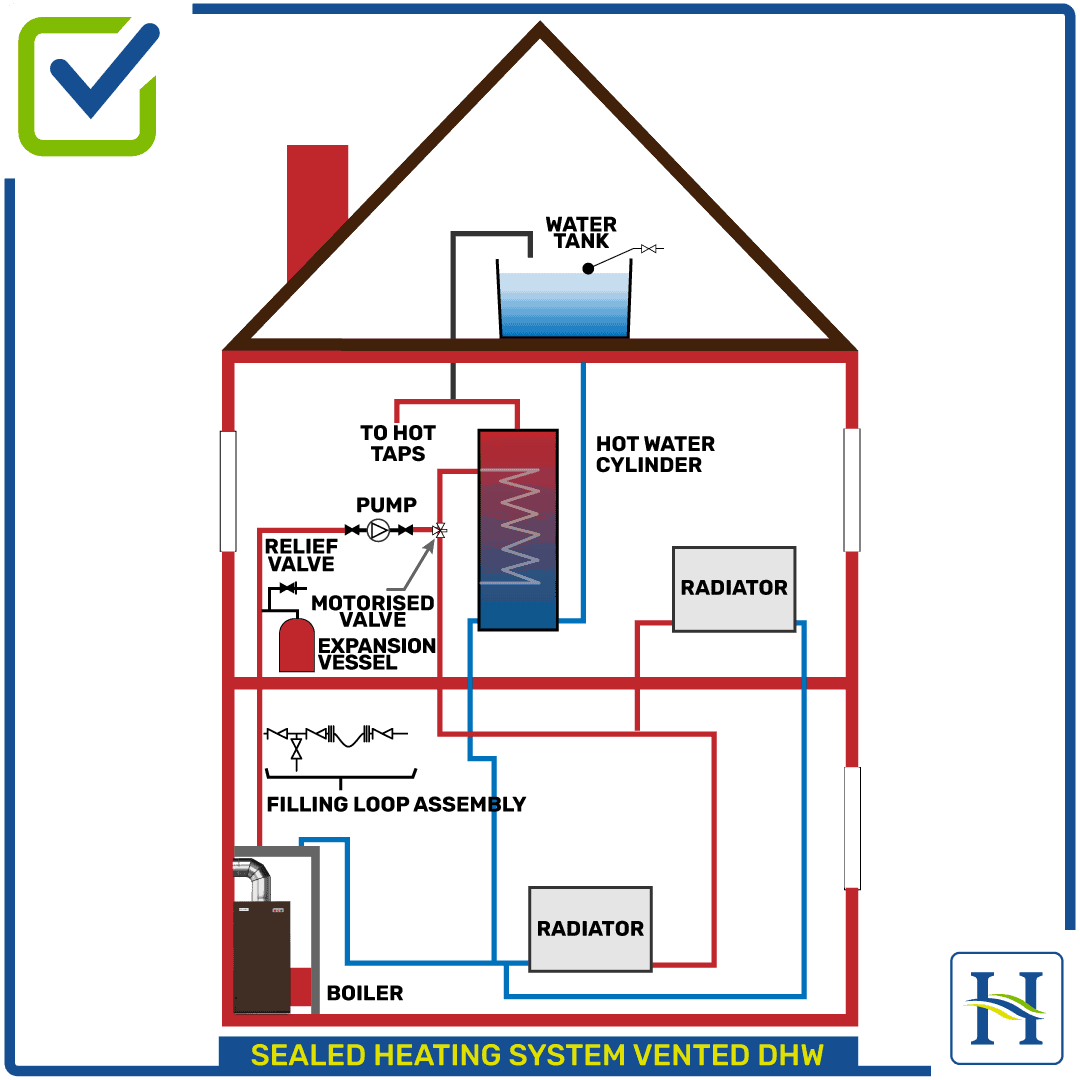
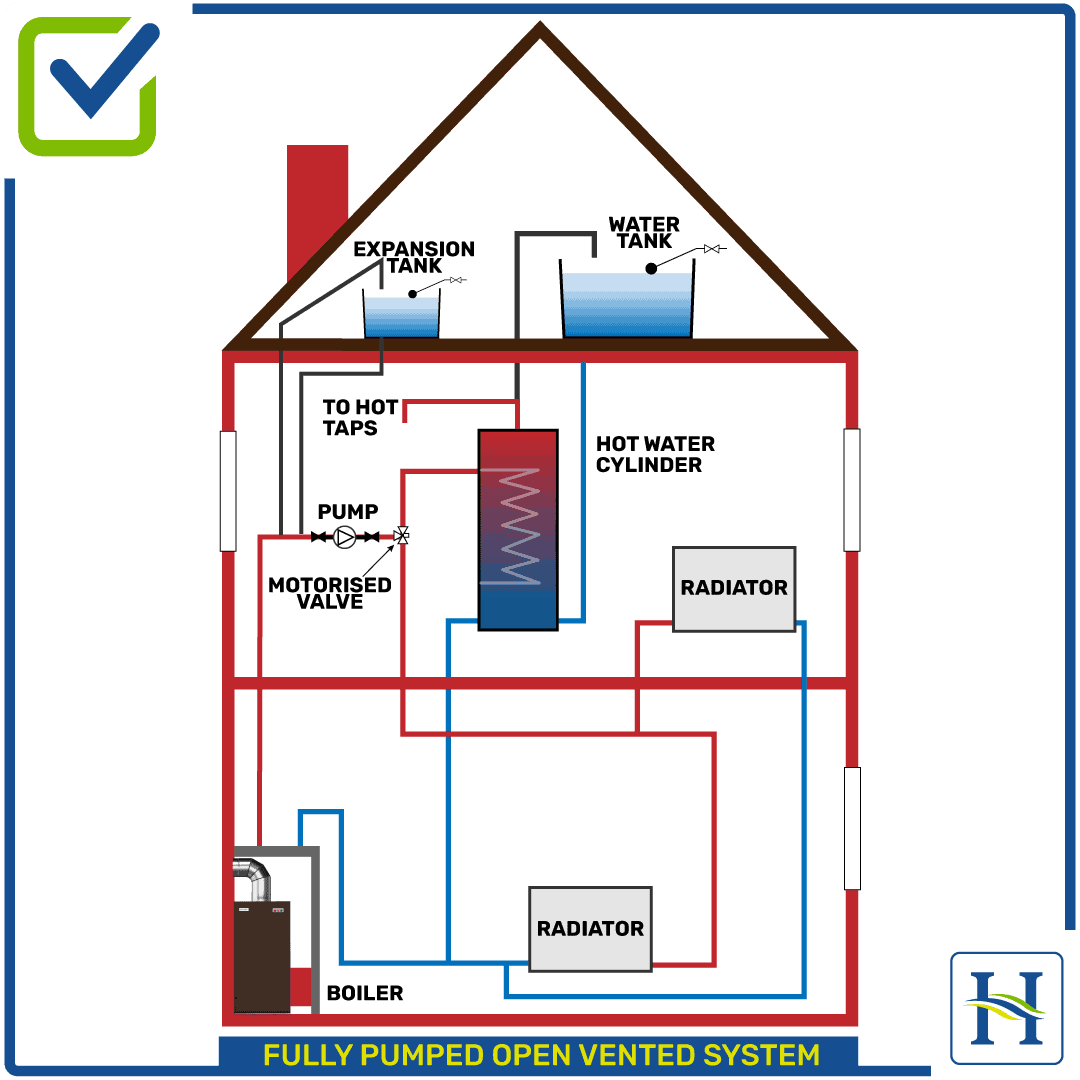
In a conventional boiler, fuel is burned and the hot gases produced are passed through a heat exchanger where much of their heat is transferred to water, thus raising the water’s temperature. One of the hot gases produced in the combustion process is water vapour (steam), which arises from burning the hydrogen content of the fuel.
Condensing boilers extracts additional heat from the waste gases by condensing the water vapour to liquid water, thus recovering its latent heat. The effectiveness of this condensing process varies, it depends upon the temperature of the water returning to the boiler, but for the same conditions, it is always at least as efficient as a non-condensing boiler.
It is normal for a condensing boiler to produce a plume of water vapour from the flue terminal; it demonstrates the boiler is working extremely efficiently and may be more prevalent when the boiler starts up. Dependent on the heating system, about 1 litre of condensate per hour is produced. Condensate will have a pH value in the range of 3.5-5, which is about the same acidity as tomato juice.